What Is A 510 Registered Manufacturer?
If yous've never prepared a medical device FDA premarket notification, usually known as a 510(k) submission, figuring out where to begin can be daunting. The FDA website provides a goldmine of information but extracting those gilded nuggets requires lots of digging. In this mail service, we'll provide a primer on how to approach the FDA 510(k) approval process, explain how the process works, and talk more about predicate device research and identifying the right product code.
Already familiar with the basic 510(k) procedure? Skip to our second post, which discusses guidance documents and data needed for submissions. Nosotros've combined all three posts into one easy to read white paper. Download it hither.
Basic Steps in the Medical Device Premarket Notification Procedure
We'll explain various steps in the procedure throughout this blog series, but let's start with a holistic review of the FDA approval process.
Ostend classification of your medical device and whether it falls under the 510(k) pathway.
| 1 | Ostend classification of your medical device and whether it falls nether the 510(k) pathway. |
| 2 | Using the FDA website, identify the appropriate three-letter product lawmaking and regulation number for your device. |
| 3 | Conduct enquiry on the FDA database and select a predicate for comparison. |
| four | Search on the FDA website for applicable FDA guidance documents. |
| 5 | Decide which international "consensus standards" may utilize to your device. |
| 6 | Identify clinical information and/or testing that may be required for your device. |
| 7 | Complete performance testing and perform clinical studies (if required). |
| viii | Assemble all documentation into the 510(k) application. |
| ix | Review the Turn down to Accept (RTA) checklist to ensure that you're following the FDA guidelines for completeness. |
| 10 | Pay the 510(chiliad) review fee, get the receipt, and and then submit the 510(k) to FDA. |
| xi | Receive confirmation from FDA within 2 weeks that your 510(yard) was accepted for substantive review. |
| 12 | If y'all 510(k) is adamant to be substantially equivalent, you lot will receive a letter and it will exist posted on the FDA website and this volition serve equally proof that your device may be legally marketed in the United states. No certificate will be issued. |
The official nomenclature for a 510(k) is premarket notification . We should annotation that FDA does not actually "corroborate" 510(k) submissions – they "clear" (authorize) a device to be marketed in the Us. That's why a 510(thou) is chosen a premarket notification and not premarket approval (PMA), which applies only to Class Three devices. Merely for the sake of clarity, we use the term approval in this introductory article. The FDA does "approve" Class Three medical devices via the PMA process.
Ostend That You Have a Medical Device That's Regulated past FDA and Needs a 510(m)
This may seem obvious, but the very first step is to ostend that your production is a regulated medical device and needs to go through the 510(k) approval procedure. Some products (i.due east., low-hazard devices Grade I products) do need to exist registered with FDA but don't need to go through the FDA 510(k) process. Most all Class Ii devices must procure a 510(grand). Following is data on how you can find out whether your device is regulated and needs a 510(k).
Identify the Right Product Code and Regulation Number for Your Medical Device
FDA uses a predicate-based review arroyo. This ways that when you lot submit your application to FDA, you will be comparing your medical device to a very like device that has already been approved (the predicate) by FDA. This process is quite different from the arroyo used in Europe or Canada – those markets apply a risk-based rules approach to device registration, which means the device must largely stand on its own merits with regard for safety and performance. Because FDA requires you to identify a unmarried predicate device, your first step will be to find one. You lot may already take a good thought of which competitive products would make a suitable predicate for comparing in your 510(k). In whatsoever case, you lot should start your research using the FDA Production Classification database.
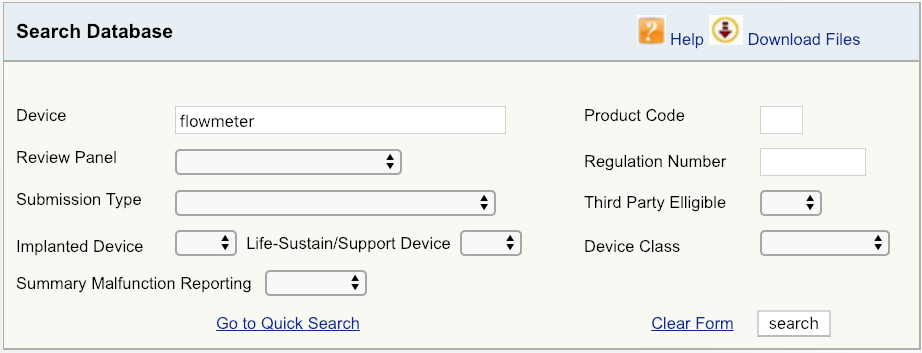
For example, permit'south say your company is introducing a new cardiovascular claret flowmeter to the US market. The kickoff step would be to begin with a uncomplicated device search on the FDA database, as shown, and then look at the options available. Start with broadest definition of your product – in this example, just the term "flowmeter." The results bear witness that there are six unique FDA production codes for products related to flowmeter.
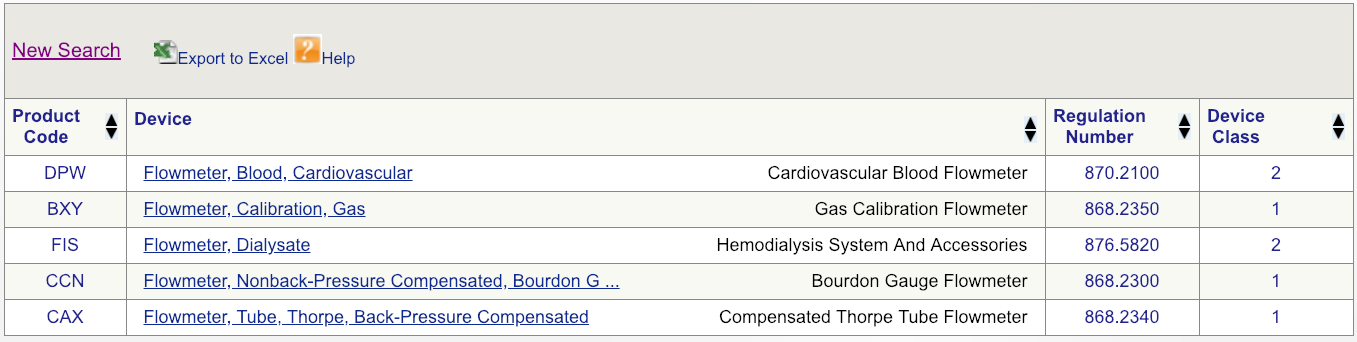
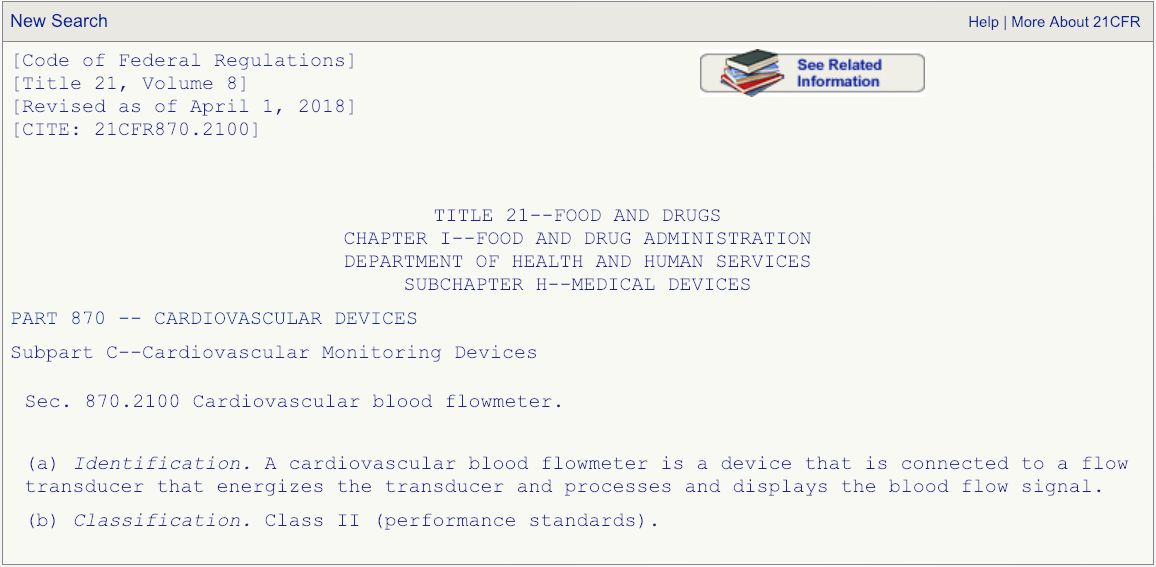
Code DPW looks to be the best friction match but, to brand certain, click on the regulation number and carefully read the description.
Choosing the Right Predicate Device for Your 510(thou) Submission
After you take read the description associated with the regulation number and are admittedly sure that the production code DPW is the correct one, and so get the FDA's 510(k) database and search for any devices cleared under production lawmaking DPW.
This is where things can get catchy and you need to be conscientious. In this case, there are 131 cleared medical devices under classification product code DPW. Which ane will make the all-time predicate for your device? Well, here's a piece of advice: When reviewing your options (hopefully you will not have 131 options), information technology is best to sort by the "Decision Date" column and beginning with devices that were cleared recently. Why? While it may be tempting to choose an older device as your comparative predicate, the FDA frowns upon using devices cleared more than 10 years agone.
Your next pace will be to click on the "Summary" link for each device every bit shown (see the example page below).
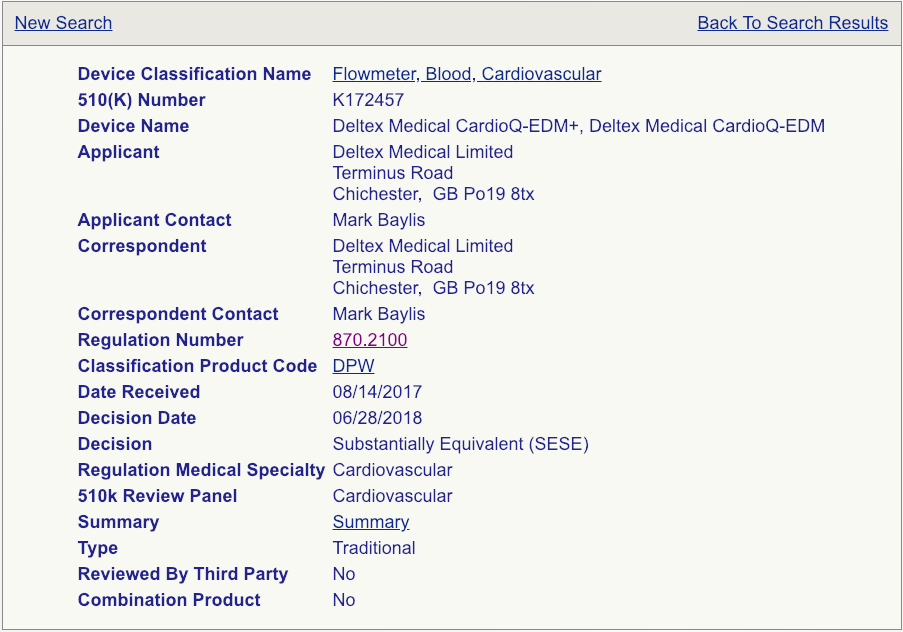
Read these summaries very, very carefully. Pay attention to the intended utilise, allowed indications for use, testing conducted, and clinical studies that may have been performed. Some 510(1000) summaries provide more than data than others, so make certain you review equally many as possible and aggregate your cognition in a spreadsheet if y'all are reviewing a lot of summaries. Your called predicate does not need to be identical to your device, simply it needs to exist shut enough non to raise additional safety and effectiveness questions. The chosen predicate must have the same intended utilize and indications for utilize. This is very important. If the indications for use are different, that device won't be a suitable predicate. The technological features should closely match your device. Choosing the correct predicate is truly critical for the success of your submission and, if you have any reservations about your options, you should seek the advice of an experienced FDA consultant.
What Happens If Your Device Is Truly Innovative?
A limitation of the FDA predicate registration system is that it does not easily arrange innovation. In the by, this was why some companies introducing innovative technology chose to introduce their devices to the European market place offset. If yous have truly new applied science or your device combines two existing technologies, you tin enquire FDA to render an stance on the classification and regulatory requirements for the device by submitting a 513(k) request for information. Some companies making innovative low-risk medical devices without a suitable predicate device tin can go through the De Novo process. This allows FDA to assign a Form I or Grade II designation and product code/regulation number to a production that has no current relevant product code.
You may also asking a Q-Submission Coming together (Q Sub) with FDA to analyze regulatory requirements, get advice on what technical documentation to include, or hash out clinical studies needed to support your submission. These curt meetings (in person, via phone, or in writing) permit you to ask a few noun questions (depending on time allowed) and tin exist a worthwhile way to ensure that you don't waste matter fourth dimension and money. Unless you lot are absolutely confident of the path you need to have and what will be required in your submission, we recommend taking reward of a Q-Sub coming together. (We can aid yous with this.) Almost ii-thirds of all FDA 510(k) submissions are slowed downward by Additional Information (AI) requests from FDA reviewers. Nosotros'll talk more near AI requests afterwards.
Want to Acquire More About the 510(k) Process?
If yous establish this article informative, read the 2nd function of this serial. We'll focus on how you can determine which guidance documents and international standards are applicable to your device. If you lot really desire to learn more than about how the 510(chiliad) procedure works prior to compiling your application, consider our training class on FDA 510(k) and CE Mark registration. Our FDA consultants can too assist with compiling or reviewing your submission.
What Is A 510 Registered Manufacturer?,
Source: https://www.orielstat.com/blog/fda-510k-process/
Posted by: bertrandbelity.blogspot.com


0 Response to "What Is A 510 Registered Manufacturer?"
Post a Comment